Identify The Conjugate Base Of The Following Acids [co(nh3)5(oh2)]3
All Chapters2hrs & 53mins0% complete2hrs & 49mins0% complete3hrs & 25mins0% complete1hr & 38mins0% complete47mins0% complete3hrs & 55mins0% complete3hrs & 47mins0% complete2hrs & 28mins0% complete2hrs & 35mins0% complete1hr & 57mins0% complete2hrs & 5mins0% complete1hr & 31mins0% complete3hrs & 40mins0% complete2hrs & 17mins0% complete2hrs & 22mins0% complete2hrs & 26mins0% complete4hrs & 42mins0% complete3hrs & 48mins0% complete1hr & 44mins0% complete2hrs & 58mins0% complete1hr & 33mins0% complete3hrs0% complete2hrs & 1min0% complete1hr & 54mins0% complete.
Voiceover In this video,we're going to be talking about conjugate acid-base pairs. We're going to introduce the idea of a conjugate acid-base pair using an example reaction. The example reaction isbetween hydrogen fluoride, or HF, and water. So hydrogen fluoride is a weak acid, and when you put it in water, it will dissociate partially. So some of the HF will dissociate, and you'll get fluoride minus ions.
And then that dissociated H plus ion. So this dissociated H plus ion will get donated to our water. So water then becomesH3O plus, or hydronium. And so this process isin dynamic equilibrium, cause it can go forward,and it can go backward, and eventually, those two rates are equal, and they're both happeningat the same time. So in this reaction, we havea couple things going on, and we're gonna think about it in terms of hydrogen ions being exchanged. So if we just look atthe hydrofluoric acid, and we look in the forward direction, our HF is becoming F minus. And it's doing that by donating or losing, so I'll put a minus for losing a proton.
So our HF loses a proton that forms our F minus or fluoride ion. And then we can look at that same process happening in the backwards reaction.
So if we look at the backwards reaction, which is also happening, the fluoride ion can pick up or accept a proton from somewhere. So it can pick up an H plus, so I'll have a plus, H plus here. And so when fluoride accepts a proton, we reform our HF.
So we can see that HF and F minus have this special relationship where you can form one or the other by losing or gaining a proton. And we can see a similar relationship between water and hydronium.
So, water here, we said water is accepting a proton from HF, so we see that water will gain a proton, and that will give us hydronium. In the reverse reaction, hydronium can lose aproton to reform water. So, minus H plus. So again we have these two species, water and hydronium, that are related to each other by having, or not having, one H plus. So in chemistry, we call these species that are related in this way conjugate acid-base pairs.
So the official definition, or my official definition ofa conjugate acid-base pair is when you have two speciesthat are related to each other. Let's see, two species thatare related to each other, related by one H plus. In this case, we have HF and F minus that are related to eachother by that one H plus.
And so HF and F minus area conjugate acid-base pair. We also have water and hydronium, which are also related by that one H plus. So water and H3O plus are alsoa conjugate acid-base pair. You can probably tell from the name, but whenever you have aconjugate acid-base pair, one thing in the pair will be an acid, and the other thing will always be a base.
The definition of which one is the acid and which one is the base comes from the Bronsted-Lowry definition of acids and bases. So the Brondsted-Lowry definition says anything that can donate an H plus, so anything that will giveaway an H plus is an acid. So we can see that, in this case, our hydrofluoric acidis acting as the acid in the conjugate acid-base pair. And that means that fluoride has to be acting as the base. And that makes sense, because the Bronsted-Lowry definition of a base is something that will accept an H plus. And that's exactly what it does in the reverse reaction. Your F minus will pick up an H plus and go back to your acid.
So we can also look at water and H3O plus. So here, water is gaininga proton, or accepting it, so water is acting as a base.
And in the reverse reaction, H3O plus is donating a proton, so H3O plus is acting as an acid. The relationship betweenconjugate acid-base pairs we can write a little bit more generally. So, if we representany generic acid as HA. So this is our acid. We said that a acid issomething that donates a proton. So it'll lose the proton, and when it does that, itwill form the conjugate base, which is represented by A minus. In the reverse reaction,our base, A minus, can gain a proton and remake our acid, or conjugate acid.
So whenever you have two species that have basically the same formula, which we abbreviated here as A minus, except for one has anH plus and one doesn't, then you know you have aconjugate acid-base pair. Fallout 4 ps4 mods list. So let's look at some more examples of conjugate acid-base pairs.
We saw above, HF, or hydrofluoric acid, it's conjugate base is F minus. So here HF is our acid, andwhen it loses that proton, we are left with F minus. We saw in the same reactionthat water can act as a base. So if water is our A minus, if that water accepts a proton, it forms the conjugate acid H3O plus.
So the example we've gone through so far, HF, is for a weak acid. But we can also talk about the conjugate base of a strong acid, like hydrochloric acid. HCl is a strong acid, so that means it completely dissociates. So it gives away all of its protons, and when it does that, we're left with theconjugate base, chloride. So even though chlorideisn't particularly basic, it's still the conjugate base of HCl.
And last but not least, we're gonna go through two examples where it looks like we might havea conjugate acid-base pair, but we actually don't. So one example is, whatabout the relationship between H3O plus and OH minus?

If we think of our acid up here being H3O plus, if we lose one proton, we saw that its conjugate base is water. If water loses anotherproton, we get OH minus. So the difference betweenthese two species here is two protons instead of one proton. So these two, hydronium and hydroxide are not a conjugate acid-base pair because they differ by twoprotons instead of one. And then the lastexample we'll look at is, we said that fluoride isa conjugate base of HF. So what about the relationship between sodium fluoride and fluoride? And so these two are alsonot a conjugate base pair because if we take our fluoride ion, and it accepts a proton, we don't get sodium fluoride.
They are related by a sodium ion. So by definition, these two are not a conjugate acid-base pair. So in this video, we learnedthat a conjugate acid-base pair is when you have two species and they have the same formula, except one has an extra proton.
Identify The Conjugate Base Of The Following Acids Co(nh3)5(oh2) 3 8
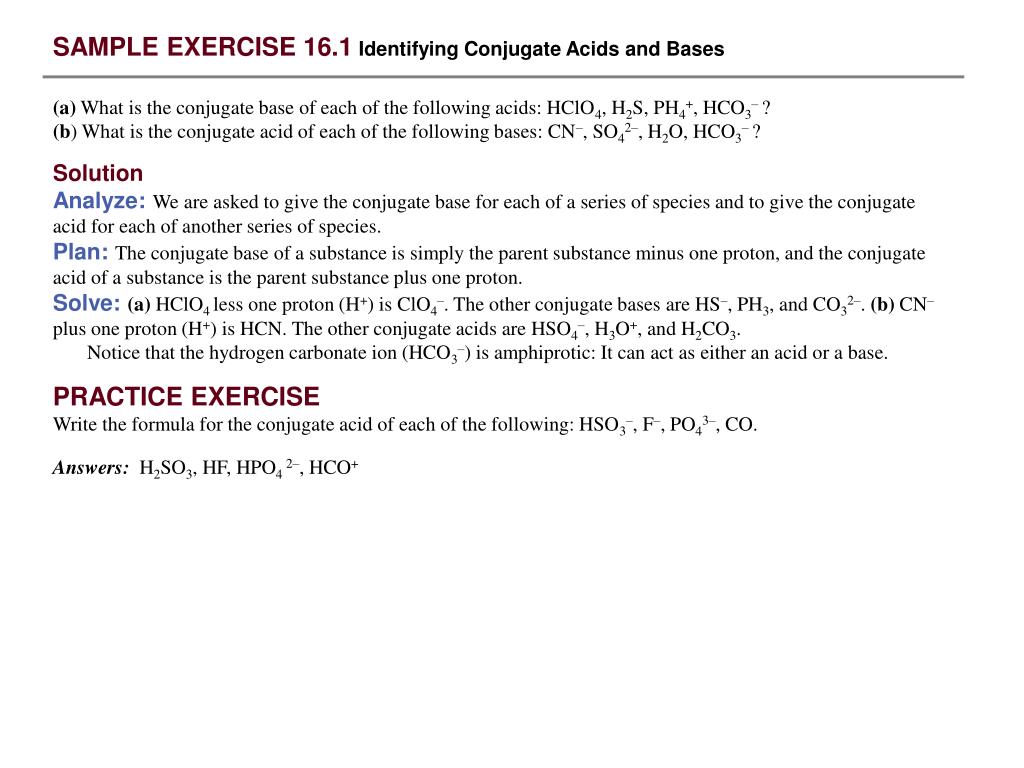
So the acid has an extra proton, which it can lose to form the base.